
The Stage 2 webinar on the rest of the standard, including but not limited to: The Stage 1 webinar focuses on the following processes:
ISO 13485 INTERNAL AUDIT CHECKLIST FREE ISO
We followed the same format, 2-part Stage 1 and Stage 2, for all of the subsequent ISO 13485 training webinars. The webinars were updated in 2016 and again in 2018. Our previous webinar on the 2003 version of ISO 13485 was split into two parts: Stage 1 and Stage 2. This 2-part webinar has been previously recorded three different times. Previous versions of the ISO 13485 webinars Our current turnkey quality system clients are located in countries all over the world, including Finland, Japan, Australia, France, and Canada. We have four qualified lead auditors as employees and three subcontractors that are ISO 13485 lead auditors. We have turnkey quality systems for the US FDA, European MDR, and MDSAP, and we are finishing our most recent turnkey system for compliance with the European IVDR. Medical Device Academy is currently helping multiple device manufacturers implement their first quality system for compliance–including SaMD, electromedical devices, implants, and IVD devices. This ISO 13485 training webinar is also included as part of our turnkey quality system. Dozens and dozens of consulting clients have successfully achieved ISO 13485 certification with Medical Device Academy’s turnkey quality system procedures, and hundreds of quality systems were audited and/or improved. In 2012, Rob began building a library of quality system procedures for a turn-key quality system and selling the procedures from the Medical Device Academy website. In 2011 his medical device blog postings began as a way to help medical device companies. In April of 2010, he purchased the URL and he began to help companies implement quality systems as a consultant (while continuing to audit and train 140 days per year for BSI). In October of 2009, after successfully managing quality systems for three different medical device manufacturers, Rob joined BSI as a Lead Auditor and instructor. Rob Packard created his first quality system in the Spring of 2004. Medical Device Academy’s experience with ISO 13485 training

The standard is available from the Estonian Centre for Standardisation and Accreditation.” Date of Availability of the European standard is. This standard has been endorsed with a notification published in the official bulletin of the Estonian Centre for Standardisation and Accreditation. “This Estonian standard EVS-EN ISO 13485:2016/A11:2021 consists of the English text of the European standard EN ISO 13485:2016/A11:2021. Here’s a copy of the text from the beginning of the Standard: This version is also referred to as A11:2021.
The only difference between the EN ISO version and the International ISO version is that the EN ISO version includes harmonization Annex ZA for compliance with the EU MDR and Annex ZB for compliance with the EU IVDR.
ISO 13485 INTERNAL AUDIT CHECKLIST FREE LICENSE
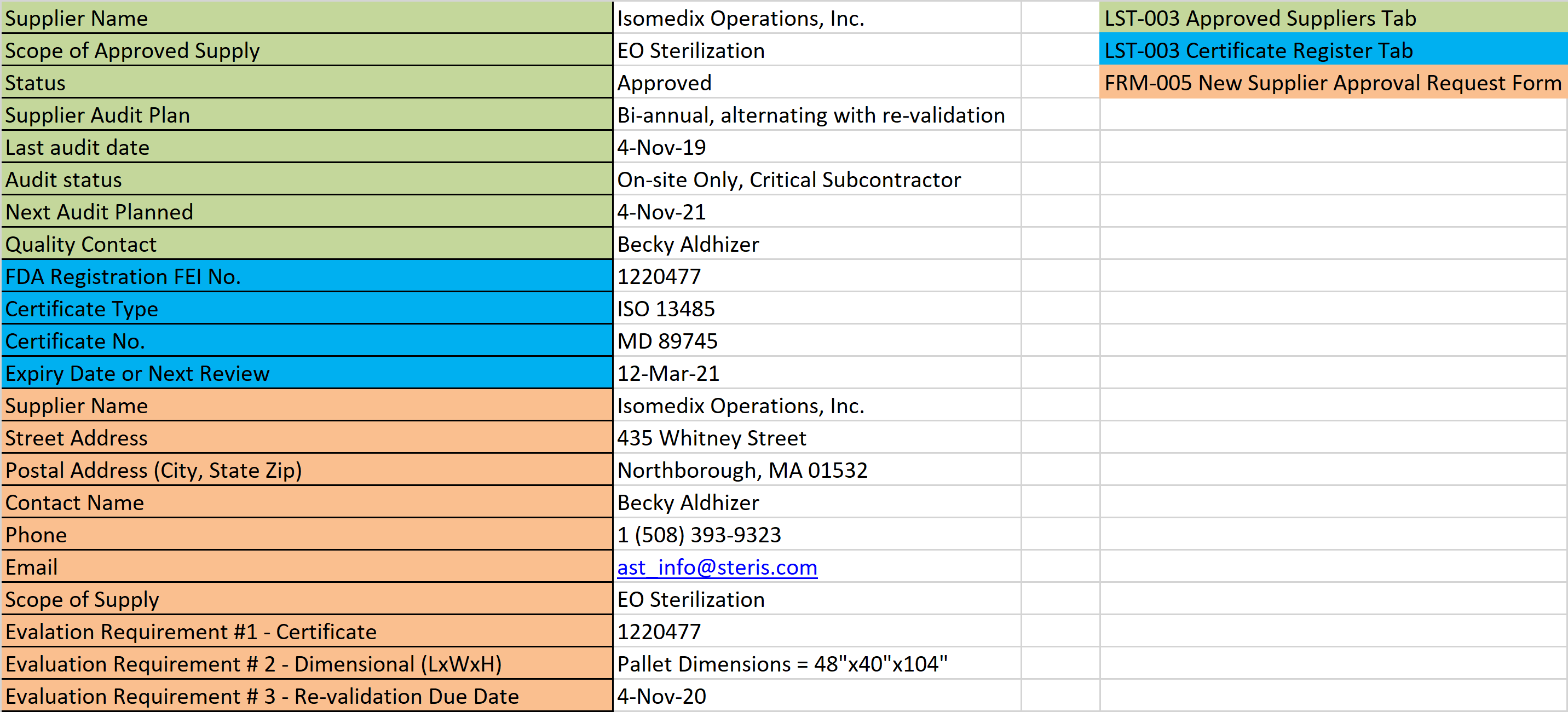
If you purchase a copy, we recommend selecting the option for a multi-user license so the standard can be used by more than one person in your company and printed. If you are looking for the best place to purchase a copy of the ISO 13485:2016 standard, we recommend the Estonian Centre for Standardisation and Accreditation. Yes, you need to maintain a copy of the ISO 13485 standard as a “document of external origin.” This is needed for reference when you are making updates to procedures in your quality system.



 0 kommentar(er)
0 kommentar(er)
